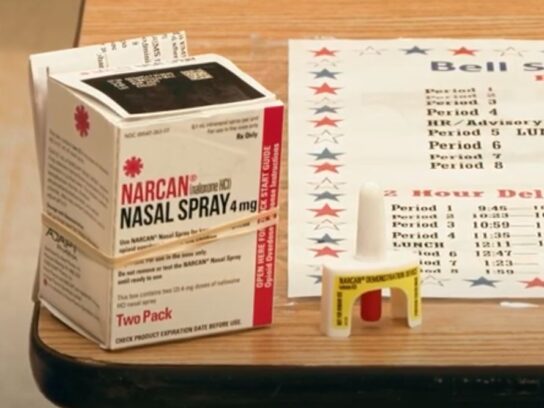
The Food and Drug Administration (FDA) approved NARCAN Nasal Spray for over-the-counter use, a big step in increasing access to the opioid overdose reversal agent.
According to Emergent BioSolutions, NARCAN is expected to be available over the counter later this summer. The decision means that anyone will be able to access NARCAN without a prescription. The decision comes as the opioid crisis escalates in the United States, with a person dying from an opioid overdose every eight minutes.
President and CEO of Emergent BioSolutions Robert G. Kramer says this decision is a historic milestone in the fight against opioids.
“We are dedicated to improving public health and assisting those working hard to end the opioid crisis,” Kramer said in a press release. “So now with leaders across government, retail and advocacy groups, we must work together to continue increasing access and availability, as well as educate the public on the risks of opioid overdoses and the value of being prepared with NARCAN Nasal Spray to help save a life.”
More than 44 million doses of NARCAN Nasal Spray have been distributed since its launch in 2016, according to Emergent BioSolutions. The transition to over-the-counter use is supported by several studies, including seven years of post-marketing safety data.
The new over-the-counter NARCAN will have the same formulation, device design and prescription strength. NARCAN can help counteract the effects of opioids, including fentanyl.
The Gaithersburg based company anticipates NARCAN to be available on shelves and online retailers by late summer.

